
Back آنمونین AZB Anemonin German Anemonina Spanish آنمونین Persian Anémonine French Anemonin Hungarian アネモニン Japanese Anemonina Polish Anemonin Serbo-Croatian Anemonin Serbian
 | |
 | |
| Names | |
|---|---|
| IUPAC names
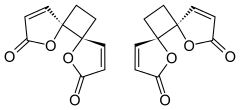
trans-4,7-Dioxadispiro[4.0.46.25]dodeca-1,9-diene-3,8-dione
trans-1,7-Dioxadispiro[4.0.4.2]dodeca-3,9-diene-2,8-dione[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.170 g·mol−1 |
| Appearance | Colourless, odourless solid |
| Density | 1.45g/cm3 |
| Melting point | 158[1] °C (316 °F; 431 K) |
| Boiling point | 535.7 °C (996.3 °F; 808.9 K) @ 760mmHg |
| low | |
| Solubility in chloroform | very soluble[1] |
| Hazards | |
| Flash point | 300.7 °C (573.3 °F; 573.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg·kg−1 (mouse, IP) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
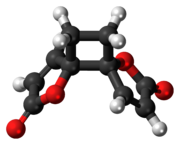
Anemonin is a tri-spirocyclic dibutenolide natural product found in members of the buttercup family (Ranunculaceae) such as Ranunculus bulbosus, R. ficaria, R. sardous, R. sceleratus,[2] and Clematis hirsutissima.[3] Originally isolated in 1792 by M. Heyer,[4] It is the dimerization product of the toxin protoanemonin.[5] One of the likely active agents in plants used in Chinese medicine as an anti-inflammatory[6] and Native American medicine as a horse stimulant,[3] its unique biological properties give it pharmaceutical potential as an anti-inflammatory and cosmetic agent.
- ^ a b c William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–26. ISBN 978-1-4987-5429-3.
- ^ Teodora N, Neli Kinga O, Daniela H, Daniela B, Pripon F, Aurel A, Claudia T (2018). "Anemonin Content of Four Different Ranunculus Species". Pakistan Journal of Pharmaceutical Sciences. 31 (5(Supplementary)): 2027–2032. PMID 30393208.
- ^ a b Kern JR, Cardellina JH (July 1983). "Native American medicinal plants. Anemonin from the horse stimulant Clematis hirsutissima". Journal of Ethnopharmacology. 8 (1): 121–123. doi:10.1016/0378-8741(83)90093-4. PMID 6632934.
- ^ Moriarty RM, Romain CR, Karle IL, Karle J (July 1965). "The Structure of Anemonin". Journal of the American Chemical Society. 87 (14): 3251–3252. doi:10.1021/ja01092a047. ISSN 0002-7863.
- ^ "Aktuelles aus der Natur" (PDF) (in German). TU Graz. 2 April 2009. p. 4. Retrieved 27 November 2010.[permanent dead link]
- ^ Duan H, Zhang Y, Xu J, Qiao J, Suo Z, Hu G, Mu X (April 2006). "Effect of anemonin on NO, ET-1 and ICAM-1 production in rat intestinal microvascular endothelial cells". Journal of Ethnopharmacology. 104 (3): 362–366. doi:10.1016/j.jep.2005.09.034. PMID 16257161.