
Back بروموبرايد Arabic Bromoprida Basque Bromopride Italian Bromoprida Portuguese Bromopridă Romanian Бромоприд Russian Bromoprid Serbo-Croatian Bromoprid Serbian
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50 to 75% (oral) 78% (intramuscular) |
| Protein binding | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 4 to 5 hours |
| Excretion | Renal, 10 to 14% unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.675 |
| Chemical and physical data | |
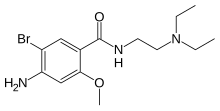
| Formula | C14H22BrN3O2 |
| Molar mass | 344.253 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bromopride (INN) is a dopamine antagonist with prokinetic properties widely used as an antiemetic, closely related to metoclopramide. It is not available in the United States.
Bromopride appears to be safe and effective for use in pregnancy.[1]
- ^ Araújo JR (1981). "Evaluation of bromopride in nausea and vomiting of pregnancy". J Bras Ginecol (in Portuguese). 91 (4): 283–5.