Back ريبوز منقوص الأكسجين Arabic Dezoksiriboza Azerbaijani دئوکسیریبوز AZB Дэзоксірыбоза Byelorussian Дезоксирибоза Bulgarian ডিঅক্সিরাইবোজ Bengali/Bangla Dezoksiriboza BS Desoxiribosa Catalan ڕایبۆزی کەم ئۆکسجین CKB Deoxyribóza Czech
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
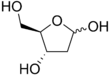
2-deoxy-d-ribose
| |||
| Other names
2-deoxy-d-erythro-pentose
thyminose | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[1] | |||
| C5H10O4 | |||
| Molar mass | 134.131 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 91 °C (196 °F; 364 K) | ||
| Very soluble | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. Discovered in 1929 by Phoebus Levene,[2] deoxyribose is most notable for its presence in DNA. Since the pentose sugars arabinose and ribose only differ by the stereochemistry at C2′, 2-deoxyribose and 2-deoxyarabinose are equivalent, although the latter term is rarely used because ribose, not arabinose, is the precursor to deoxyribose.
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.). Merck. 1989. ISBN 091191028X., 2890
- ^ "Comprehensive Timeline of Biological Discoveries" (PDF). Archived from the original (PDF) on 10 September 2016. Retrieved 31 July 2017.