
Back فينيرينون Arabic Finerenon German Φινερενόνη Greek Finérénone French Finerenone ID Finerenone Italian Финеренон Macedonian ଫାଇନରନନ OR Finerenona Portuguese Финеренон Russian
 | |
| Clinical data | |
|---|---|
| Trade names | Kerendia |
| Other names | BAY 94-8862 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a621038 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral |
| Drug class | Potassium-sparing diuretic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.614 |
| Chemical and physical data | |
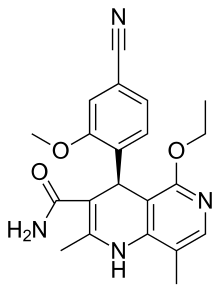
| Formula | C21H22N4O3 |
| Molar mass | 378.432 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Finerenone, marketed under the brand name Kerendia among others, is a medication used to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adults with chronic kidney disease associated with type 2 diabetes.[8] Finerenone is a non-steroidal mineralocorticoid receptor antagonist (MRA).[7] It is taken orally (swallowed by mouth).
Common side effects include hyperkalemia (high levels of potassium) (normal 3.5-5.5mg/dl), hypotension (low blood pressure), and hyponatremia (low levels of sodium).[8]
Finerenone was approved for medical use in the United States in July 2021,[8][10] and in the European Union in February 2022.[9] The US Food and Drug Administration considers it to be a first-in-class medication.[11]
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Retrieved 13 May 2022.
- ^ a b "Kerendia APMDS". Therapeutic Goods Administration (TGA). 9 December 2021. Retrieved 12 June 2022.
- ^ "AusPAR: Finerenone". Therapeutic Goods Administration (TGA). 31 May 2022. Retrieved 12 June 2022.[permanent dead link]
- ^ "Kerendia Product information". Health Canada. 25 April 2012. Retrieved 2 January 2023.
- ^ "Kerendia Summary Basis of Decision". Health Canada. 23 October 2014. Retrieved 10 March 2023.[permanent dead link]
- ^ "Details for: Kerendia". Health Canada. 22 November 2022. Retrieved 3 March 2024.
- ^ a b "Kerendia- finerenone tablet, film coated". DailyMed. Retrieved 20 August 2021.
- ^ a b c d "FDA Approves Drug for Chronic Kidney Disease". U.S. Food and Drug Administration (FDA). 9 July 2021. Retrieved 9 July 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Kerendia EPAR". European Medicines Agency (EMA). 14 December 2021. Retrieved 11 March 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Bayer's Kerendia (finerenone) Receives U.S. FDA Approval for Treatment of Patients with Chronic Kidney Disease Associated with Type 2 Diabetes" (Press release). Bayer. 9 July 2021. Retrieved 9 July 2021 – via Business Wire.
- ^ Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (PDF). U.S. Food and Drug Administration (FDA) (Report). 13 May 2022. Archived from the original on 6 December 2022. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.