
Back فوندابارينوكس Arabic Fondaparinux German Φονταπαρινόξη Greek Fondaparinux Spanish فونداپارینوکس Persian Fondaparinux French Fondaparinuks ID Fondaparinux Italian フォンダパリヌクス Japanese 폰다파리눅스 Korean
 | |
| Clinical data | |
|---|---|
| Trade names | Arixtra |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 94% |
| Metabolism | renally excreted unchanged |
| Elimination half-life | 17-21 hours[1] |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
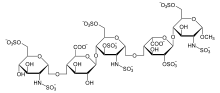
| Formula | C31H43N3Na10O49S8 |
| Molar mass | 1728.03 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fondaparinux (trade name Arixtra) is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.
- ^ Walenga JM, Jeske WP, Fareed J (2005). "Biochemical and Pharmacologic Rationale for Synthetic Heparin Polysaccharides". Chemistry and Biology of Heparin and Heparan Sulfate. Elsevier. pp. 143–177. doi:10.1016/b978-008044859-6/50006-x. ISBN 978-0-08-044859-6.
The elimination half-life of AT-bound fondaparinux is 17–21 h (171,172). The subcutaneous bioavailability of fondaparinux is nearly 100% and it is distributed mainly in the blood (165,173).