
Back إيزوليوسين Arabic İzolösin Azerbaijani ایزولوسین AZB Ізалейцын Byelorussian Изолевцин Bulgarian আইসোলিউসিন Bengali/Bangla Izoleucin BS Isoleucina Catalan Isoleucin Czech Isoleucin Danish
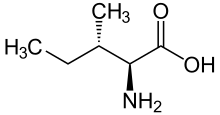 Skeletal formula of L-isoleucine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
isoleucine
| |||
| Other names
(2S,3S)-2-amino-3-methylpentanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.726 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| −84.9·10−6 cm3/mol | |||
| Supplementary data page | |||
| Isoleucine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
isoleucine (symbol Ile or I)[1] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine.[2] In plants and bacteria, isoleucine is synthesized from a pyruvate employing leucine biosynthesis enzymes.[3] It is encoded by the codons AUU, AUC, and AUA.
- ^ "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations 1983". The Biochemical Journal. 219 (2): 345–373. April 1984. doi:10.1042/bj2190345. PMC 1153490. PMID 6743224.
- ^ Joshi V, Joung JG, Fei Z, Jander G (October 2010). "Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress". Amino Acids. 39 (4): 933–947. doi:10.1007/s00726-010-0505-7. PMID 20186554. S2CID 22641155.
- ^ Kisumi M, Komatsubara S, Chibata I (July 1977). "Pathway for isoleucine formation form pyruvate by leucine biosynthetic enzymes in leucine-accumulating isoleucine revertants of Serratia marcescens". Journal of Biochemistry. 82 (1): 95–103. doi:10.1093/oxfordjournals.jbchem.a131698. PMID 142769.

