
Back ملانوتان II AZB Melanotan Danish Melanotan II German Melanotan II Spanish ملانوتان II Persian Melanotan II Dutch Melanotan NB Меланотан 2 SAH Melanotan II Serbo-Croatian Melanotan II Serbian
 | |
| Names | |
|---|---|
| Pronunciation | /mɛˈlænoʊtæn/ ⓘ |
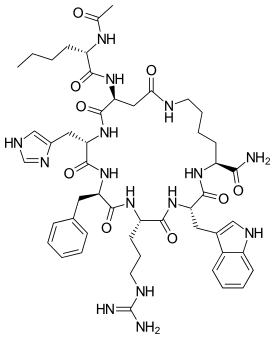
| Systematic IUPAC name
L-Lysinamide, N-acetyl-L-norleucyl-L-alpha-aspartyl-L-histidyl-D-phenylalanyl-L-arginyl-L-tryptophyl-, cyclic (2-7)-peptide | |
| Other names
List of other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | melanotan-II |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C50H69N15O9 | |
| Molar mass | 1024.180 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Melanotan II is a synthetic analogue of the peptide hormone α-melanocyte-stimulating hormone (α-MSH) that stimulates melanogenesis to facilitate tanning. It may also increase sexual arousal.
It was developed as a successor to afamelanotide ("Melanotan I"), an FDA approved drug operating through a similar pathway. Clinuvel Pharmaceuticals intended to offer it as a cosmetic, but abandoned this pursuit in the 2000s due to regulatory restrictions and concerns about the promotion of suntanning. Unlicensed Melanotan II is found on the internet, although health agencies advise against its use due to legality and a lack of testing.
Melanotan-II may cause reversible darkening of moles and freckles. It is unclear if Melanotan II can increase (or reduce) the risk of developing melanoma, because reports of melanomas associated with its use have coincided with heavy UV exposure and sun bed use.[1][2] A 2013 scientific review found there was no conclusive evidence it causes melanoma,[1] and a 2021 review concluded "the increased risk of melanoma in Melanotan users, who use it for tanning and exhibit sun-seeking behaviour, can probably be explained by more UV exposure".[2]
Side effects may include facial flushing, nausea and erection in males.
- ^ a b Javed, Muhammad; Yarrow, Jeremy; Hemington gorse, Sarah (2013-10-01). "Does melanotan injections (TAN JAB) cause melanoma? A systemic review of the effects of melanotan injections". International Journal of Surgery. 11 (8): 677. doi:10.1016/j.ijsu.2013.06.485. ISSN 1743-9191.
- ^ a b Wensink, Debby; Wagenmakers, Margreet A.E.M.; Langendonk, Janneke G. (2021-02-01). "Afamelanotide for prevention of phototoxicity in erythropoietic protoporphyria". Expert Review of Clinical Pharmacology. 14 (2): 151–160. doi:10.1080/17512433.2021.1879638. ISSN 1751-2433. PMID 33507118.