
Back كلوريد النتروزيل Arabic نیتروسیل کولورید AZB Clorur de nitrosil Catalan Chlorid nitrosylu Czech Nitrosylchlorid German Nitrozila klorido Esperanto Cloruro de nitrosilo Spanish نیتروسیل کلرید Persian Nitrosyylikloridi Finnish Chlorure de nitrosyle French
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Nitrosyl chloride[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.018.430 |
| EC Number |
|
| E number | E919 (glazing agents, ...) |
| MeSH | nitrosyl+chloride |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1069 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
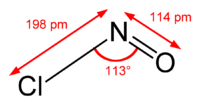
| NOCl | |
| Molar mass | 65.459 g mol−1 |
| Appearance | yellow gas |
| Density | 2.872 mg mL−1 |
| Melting point | −59.4 °C (−74.9 °F; 213.8 K) |
| Boiling point | −5.55 °C (22.01 °F; 267.60 K) |
| Reacts | |
| Structure | |
| Dihedral, digonal | |
| Hybridisation | sp2 at N |
| 1.90 D | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
261.68 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
51.71 kJ mol−1 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | inchem.org |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.[1]
- ^ Tilden, William A. (1874). "XXXII.—On aqua regia and the nitrosyl chlorides". J. Chem. Soc. 27: 630–636. doi:10.1039/JS8742700630.
