
Back Norethynodrel German Noretinodrel Spanish Noretinodrel Serbo-Croatian Noretinodrel Serbian 异炔诺酮 Chinese
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Enovid (with mestranol), others |
| Other names | Norethynodrel; Noretinodrel Norethinodrel; NYD; SC-4642; NSC-15432; 5(10)-Norethisterone; 17α-Ethinyl-19-nor-5(10)-testosterone; 17α-Ethynyl-δ5(10)-19-nortestosterone; 17α-Ethynylestr-5(10)-en-17β-ol-3-one; 19-Nor-17α-pregn-5(10)-en-20-yn-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Estrogen |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Noretynodrel: to albumin and not to SHBG or CBG[1] |
| Metabolism | Liver, intestines (hydroxylation, isomerization, conjugation)[1][3] |
| Metabolites | • 3α-Hydroxynoretynodrel[2] • 3β-Hydroxynoretynodrel[2] • Norethisterone[2][1][3] • Ethinylestradiol[3][4]• Conjugates[3] |
| Elimination half-life | Very short (< 30 minutes)[5] |
| Excretion | Breast milk: 1%[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.620 |
| Chemical and physical data | |
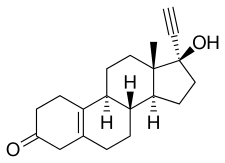
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed.[3][6][7][8] It was available both alone and in combination with an estrogen.[7][8][9] The medication is taken by mouth.[7]
Noretynodrel is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[3] It is a relatively weak progestogen.[10] The medication has weak estrogenic activity, no or only very weak androgenic activity, and no other important hormonal activity.[3][8][11][12] It is a prodrug of various active metabolites in the body, such as norethisterone among others.[3][13]
Noretynodrel was introduced for medical use in 1957.[8] It was specifically approved at this time in combination with mestranol for the treatment of gynecological and menstrual disorders.[8] Subsequently, in 1960, this formulation was approved for use as a birth control pill.[8][14] It was the first birth control pill to be introduced, and was followed by birth control pills containing norethisterone and other progestins shortly thereafter.[8][14][15] Due to its nature as a relatively weak progestogen, noretynodrel is no longer used in medicine.[10] As such, it is no longer marketed.[6][16]
- ^ a b c Cite error: The named reference
pmid2170822was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
pmid22210085was invoked but never defined (see the help page). - ^ a b c d e f g h Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ Cite error: The named reference
Kuhl2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid2256526was invoked but never defined (see the help page). - ^ a b c Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 2120–2121. ISBN 978-0-85369-840-1.
- ^ a b c Jucker E, ed. (21 December 2013). Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 85–88. ISBN 978-3-0348-7065-8.
- ^ a b c d e f g Marks L (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 74–75. ISBN 978-0-300-16791-7.
- ^ Cite error: The named reference
WHO1974was invoked but never defined (see the help page). - ^ a b Williams DA, Foye WO, Lemke TL (January 2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 700–. ISBN 978-0-683-30737-5.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 36–. ISBN 978-3-642-73790-9.
- ^ Sloane E (2002). Biology of Women. Cengage Learning. pp. 426–. ISBN 978-0-7668-1142-3.
- ^ Cite error: The named reference
pmid12215716was invoked but never defined (see the help page). - ^ a b Hollinger MA (19 October 2007). Introduction to Pharmacology, Third Edition. CRC Press. pp. 160–. ISBN 978-1-4200-4742-4.
- ^ Cite error: The named reference
Ravina2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page).