
Back بنية البيروفسكيت Arabic Perowskit#Kristallstruktur German Perovskita (estructura) Spanish Perovskiit Estonian Perovskita (egitura) Basque ساختار پروسکایت Persian Perovskiittirakenne Finnish Pérovskite (structure) French פרובסקיט (מבנה) HE Perovszkit (szerkezet) Hungarian
It has been suggested that this article be merged with Antiperovskite. (Discuss) Proposed since February 2025. |
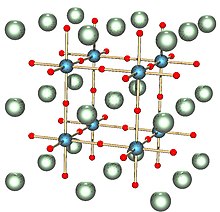


A perovskite is a crystalline material of formula ABX3 with a crystal structure similar to that of the mineral perovskite, this latter consisting of calcium titanium oxide (CaTiO3).[2] The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). In addition to being one of the most abundant structural families, perovskites wide-ranging properties and applications.[3]
- ^ A. Navrotsky (1998). "Energetics and Crystal Chemical Systematics among Ilmenite, Lithium Niobate, and Perovskite Structures". Chem. Mater. 10 (10): 2787. doi:10.1021/cm9801901.
- ^ Wenk, Hans-Rudolf; Bulakh, Andrei (2004). Minerals: Their Constitution and Origin. New York, NY: Cambridge University Press. ISBN 978-0-521-52958-7.
- ^ Artini, Cristina (2017-02-01). "Crystal chemistry, stability and properties of interlanthanide perovskites: A review". Journal of the European Ceramic Society. 37 (2): 427–440. doi:10.1016/j.jeurceramsoc.2016.08.041. ISSN 0955-2219.