
Back فينيتوين Arabic فنی توئین AZB Fenitoïna Catalan Ffenytoin Welsh Phenytoin German Φαινυτοΐνη Greek Fenitoína Spanish Fenitoina Basque فنیتوئین Persian Fenytoiini Finnish
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /fəˈnɪtoʊɪn, ˈfɛnɪtɔɪn/ |
| Trade names | Dilantin, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682022 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Anticonvulsant |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70–100% (oral), 24.4% (rectal) |
| Protein binding | 95%[3] |
| Metabolism | Liver |
| Onset of action | 10–30 min (intravenous)[4] |
| Elimination half-life | 10–22 hours[3] |
| Duration of action | 24 hours[4] |
| Excretion | Urinary (23–70%), bile[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.298 |
| Chemical and physical data | |
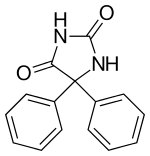
| Formula | C15H12N2O2 |
| Molar mass | 252.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phenytoin (PHT), sold under the brand name Dilantin among others,[1] is an anti-seizure medication.[3] It is useful for the prevention of tonic-clonic seizures (also known as grand mal seizures) and focal seizures, but not absence seizures.[3] The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines.[3] It may also be used for certain heart arrhythmias or neuropathic pain.[3] It can be taken intravenously or by mouth.[3] The intravenous form generally begins working within 30 minutes and is effective for roughly 24 hours.[4] Blood levels can be measured to determine the proper dose.[3]
Common side effects include nausea, stomach pain, loss of appetite, poor coordination, increased hair growth, and enlargement of the gums.[3] Potentially serious side effects include sleepiness, self harm, liver problems, bone marrow suppression, low blood pressure, toxic epidermal necrolysis,[3] and atrophy of the cerebellum.[6][7][8] There is evidence that use during pregnancy results in abnormalities in the baby.[3] It appears to be safe to use when breastfeeding.[3] Alcohol may interfere with the medication's effects.[3]
Phenytoin was first made in 1908 by the German chemist Heinrich Biltz and found useful for seizures in 1936.[9][10] It is on the World Health Organization's List of Essential Medicines.[11] Phenytoin is available as a generic medication.[12] In 2020, it was the 260th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[13][14]
- ^ a b Cite error: The named reference
genericnameswas invoked but never defined (see the help page). - ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d e f g h i j k l m "Phenytoin". The American Society of Health-System Pharmacists. Archived from the original on 8 September 2015. Retrieved 22 August 2015.
- ^ a b c Marx JA (2010). Rosen's emergency medicine: concepts and clinical practice (7th ed.). Philadelphia: Mosby/Elsevier. p. 1352. ISBN 9780323054720.
- ^ Parker KD, Elliott HW, Wright JA, Nomof N, Hine CH (March 1970). "Blood and urine concentrations of subjects receiving barbiturates, meprobamate, glutethimide, or diphenylhydantoin". Clinical Toxicology. 3 (1). Informa UK Limited: 131–145. doi:10.3109/15563657008990108. PMID 5520387.
- ^ Algahtani H, Shirah B, Alqahtani AJ, Al-Malki AQ (December 2020). "Irreversible Cerebellar Atrophy as a Complication of Short-Term Phenytoin Exposure: Clinical Improvement Following Discontinuation of the Culprit". Journal of Epilepsy Research. 10 (2): 96–99. doi:10.14581/jer.20016. PMC 7903046. PMID 33659203.
- ^ Ferner R, Day R, Bradberry SM (July 2022). "Phenytoin and damage to the cerebellum - a systematic review of published cases". Expert Opinion on Drug Safety. 21 (7): 957–977. doi:10.1080/14740338.2022.2058487. PMID 35325581.
- ^ Baba Y, Gaillard F (10 April 2017), "Phenytoin cerebellar degeneration", Radiopaedia.org, doi:10.53347/rid-52506, retrieved 22 December 2024
- ^ Aicardi J (2008). Epilepsy : a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1431. ISBN 9780781757775.
- ^ Wolfson AB (2010). Harwood-Nuss' clinical practice of emergency medicine (5th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. p. 1415. ISBN 9780781789431. Retrieved 9 June 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton RJ (2013). Tarascon pocket pharmacopoeia (14 ed.). Burlington, MA.: Jones & Bartlett Learning. p. 294. ISBN 9781449673635.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Phenytoin - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.