
Back بولي رباعي فلورو الإيثيلين Arabic تفلون AZB Тефлон Bulgarian টেফলন Bengali/Bangla Politetrafluoroetilen BS Tefló Catalan Polytetrafluorethylen Czech Тефлон CV Teflon Danish Polytetrafluorethylen German
| |
| |
| Names | |
|---|---|
| IUPAC name
Poly(1,1,2,2-tetrafluoroethylene)[1]
| |
| Other names
Fluon, Poly(tetrafluoroethene), Poly(tetrafluoroethylene), Teflon
| |
| Identifiers | |
| Abbreviations | PTFE |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.120.367 |
| KEGG | |
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
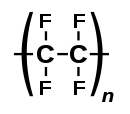
| (C2F4)n | |
| Density | 2200 kg/m3 |
| Melting point | 327 °C |
| Electrical resistivity | 1018 Ω·cm[a] |
| Thermal conductivity | 0.25 W/(m·K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert.[3] The commonly known brand name of PTFE-based composition is Teflon by Chemours,[4] a spin-off from DuPont, which originally discovered the compound in 1938.[4] Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid.
Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubricant, PTFE reduces friction, wear, and energy consumption of machinery. It is used as a graft material in surgery and as a coating on catheters.
PTFE and chemicals used in its production are some of the best-known and widely applied PFAS,[5] which are persistent organic pollutants. PTFE occupies more than half of all fluoropolymer production, followed by polyvinylidene fluoride (PVdF).[5]
For decades, DuPont used perfluorooctanoic acid (PFOA, or C8) during production of PTFE, later discontinuing its use due to legal actions over ecotoxicological and health effects of exposure to PFOA.[6][7] Dupont's spin-off Chemours today manufactures PTFE using an alternative chemical it calls GenX, another PFAS. Although GenX was designed to be less persistent in the environment compared to PFOA, it has proven to be a "regrettable substitute".[8] Its effects may be equally harmful or even more detrimental than those of the chemical it was meant to replace.[8][9][10]
- ^ "poly(tetrafluoroethylene) (CHEBI:53251)". ebi.ac.uk. Retrieved 12 July 2012.
- ^ "PTFE". Microwaves101. Archived from the original on 16 July 2014. Retrieved 16 February 2012.
- ^ "Ask a Toxicologist: Is it safe to use Teflon pans? – the Pipettepen". The Pipettepen. 6 July 2015. Retrieved 22 December 2024.
- ^ a b "The History of Teflon Fluoropolymers". Teflon.com. Retrieved 3 February 2021.
- ^ a b Améduri, B.; Sawada, Hideo, eds. (2017). Fluorinated polymers. RSC polymer chemistry series. Cambridge, UK: Royal Society of Chemistry. ISBN 978-1-78262-917-7. OCLC 951763265.[page needed]
- ^ Rich, Nathaniel (6 January 2016). "The Lawyer Who Became DuPont's Worst Nightmare". The New York Times. Retrieved 14 October 2024.
- ^ Gillam, Carey (2 June 2023). "Top US chemical firms to pay $1.2bn to settle water contamination lawsuits". the Guardian. Retrieved 14 October 2024.
- ^ a b Ahearn, Ashley (24 March 2019). "A Regrettable Substitute: The Story of GenX". Podcasts: The Researcher's Perspective. 2019 (1). doi:10.1289/EHP5134.
- ^ "US EPA deems two GenX PFAS chemicals more toxic than PFOA". Chemical & Engineering News. Retrieved 14 October 2024.
- ^ "Fact Sheet: Human Health Toxicity Assessment for GenX Chemicals" (PDF). United States Environmental Protection Agency. March 2023. Retrieved 14 October 2024.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
