
Back بيريودات البوتاسيوم Arabic پوتاسیوم پریدات AZB পটাসিয়াম পারআয়োডেট Bengali/Bangla Kaliumperiodat German Kalia perjodato Esperanto Peryodato de potasio Spanish پتاسیم پریدات Persian Periodate de potassium French Kalium periodat ID Periodato di potassio Italian
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium periodate
| |
| Other names
potassium metaperiodate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.269 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
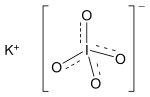
| KIO4 | |
| Molar mass | 230.00 g mol−1 |
| Appearance | white crystalline powder |
| Odor | odourless |
| Density | 3.618 g/cm3 |
| Melting point | 582 °C (1,080 °F; 855 K) (decomposes) |
| 0.17 g/100 mL (0 °C) 0.42 g/100 mL (20 °C) 4.44 g/100 mL (80 °C) 7.87 g/100 mL (100 °C) | |
| Structure | |
| tetragonal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidant |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Potassium iodide Potassium iodate |
Other cations
|
Sodium periodate |
Related compounds
|
Periodic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium periodate is an inorganic salt with the molecular formula KIO4. It is composed of a potassium cation and a periodate anion and may also be regarded as the potassium salt of periodic acid. Note that the pronunciation is per-iodate, not period-ate.
Unlike other common periodates, such as sodium periodate and periodic acid, it is only available in the metaperiodate form; the corresponding potassium orthoperiodate (K5IO6) has never been reported.
