
Back بيرين (كيمياء) Arabic Pirè Catalan Pyren Czech Pyren German Pireno Spanish پیرن (ترکیب آلی) Persian Pyreeni Finnish Pyrène (chimie) French Pirén Hungarian Pirene Italian
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pyrene[1] | |
| Other names
Benzo[def]phenanthrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1307225 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.481 |
| 84203 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H10 | |
| Molar mass | 202.256 g·mol−1 |
| Appearance | colorless solid
(yellow impurities are often found at trace levels in many samples). |
| Density | 1.271 g/cm3[2] |
| Melting point | 150.62 °C (303.12 °F; 423.77 K)[2] |
| Boiling point | 394 °C (741 °F; 667 K)[2] |
| 0.049 mg/L (0 °C) 0.139 mg/L (25 °C) 2.31 mg/L (75 °C)[3] | |
| log P | 5.08[4] |
| Band gap | 2.02 eV[5] |
| -147·10−6 cm3/mol[6] | |
| Structure[7] | |
| Monoclinic | |
| P21/a | |
a = 13.64 Å, b = 9.25 Å, c = 8.47 Å α = 90°, β = 100.28°, γ = 90°
| |
Formula units (Z)
|
4 |
| Thermochemistry[8] | |
Heat capacity (C)
|
229.7 J/(K·mol) |
Std molar
entropy (S⦵298) |
224.9 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
125.5 kJ·mol−1 |
Enthalpy of fusion (ΔfH⦵fus)
|
17.36 kJ·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
irritant |
| GHS labelling:[9] | |
 
| |
| Warning | |
| H315, H319, H335, H410 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Related PAHs
|
benzopyrene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
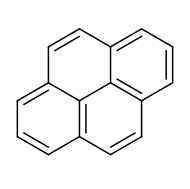
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow-green solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds.[10]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 206. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c Haynes, p. 3.472
- ^ Haynes, p. 5.162
- ^ Haynes, p. 5.176
- ^ Haynes, p. 12.96
- ^ Haynes, p. 3.579
- ^ Camerman, A.; Trotter, J. (1965). "The crystal and molecular structure of pyrene". Acta Crystallographica. 18 (4): 636–643. doi:10.1107/S0365110X65001494.
- ^ Haynes, pp. 5.34, 6.161
- ^ GHS: PubChem
- ^ Figueira-Duarte, Teresa M.; Müllen, Klaus (2011). "Pyrene-Based Materials for Organic Electronics". Chemical Reviews. 111 (11): 7260–7314. doi:10.1021/cr100428a. PMID 21740071.
