
Back Rutenium(IV)oksied Afrikaans أكسيد الروثينيوم الرباعي Arabic اوکسید روتنیوم (IV) AZB Oxid rutheničitý Czech Ruthenium(IV)-oxid German Dióxido de rutenio Spanish اکسید روتنیوم (IV) Persian Ruteniumdioksidi Finnish Dioxyde de ruthénium French Diossido di rutenio Italian
 | |
| Names | |
|---|---|
| IUPAC name
Ruthenium(IV) oxide
| |
| Other names
Ruthenium dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.660 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| RuO2 | |
| Molar mass | 133.0688 g/mol |
| Appearance | blue-black solid |
| Density | 6.97 g/cm3 |
| Boiling point | 1,200 °C (2,190 °F; 1,470 K) sublimates |
| insoluble | |
| +162.0·10−6 cm3/mol | |
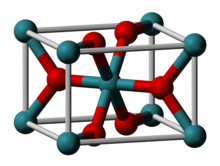
| Structure | |
| Rutile (tetragonal), tP6 | |
| P42/mnm, No. 136 | |
| Octahedral (RuIV); trigonal planar (O2−) | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Ruthenium disulfide |
Other cations
|
Osmium(IV) oxide |
| Ruthenium tetroxide | |
| Supplementary data page | |
| Ruthenium(IV) oxide (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ruthenium(IV) oxide is the inorganic compound with the formula RuO2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2.[1] Like many dioxides, RuO2 adopts the rutile structure.[2][3]
- ^ Mills, Andrew (1989). "Heterogeneous redox catalysts for oxygen and chlorine evolution". Chemical Society Reviews. 18. Royal Society of Chemistry (RSC): 285. doi:10.1039/cs9891800285. ISSN 0306-0012.
- ^ Wyckoff, R.W.G.. Crystal Structures, Vol. 1. Interscience, John Wiley & Sons: 1963.
- ^ Wells, A. F. (1975), Structural Inorganic Chemistry (4th ed.), Oxford: Clarendon Press