
Back كلوريد الساماريوم الثلاثي Arabic کولورید ساماریوم (III) AZB Chlorid samaritý Czech Samarium(III)-chlorid German کلرید ساماریوم (III) Persian Chlorure de samarium(III) French Samarium(III) klorida ID Tricloruro di samario Italian 塩化サマリウム(III) Japanese Clorură de samariu (III) Romanian
 | |
 | |
| Names | |
|---|---|
| IUPAC name
samarium(III) chloride
| |
| Other names
samarium trichloride
trichlorosamarium | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.712 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SmCl3 | |
| Molar mass | 256.76 g/mol (anhydrous) 364.80 g/mol (hexahydrate) |
| Appearance | pale yellow solid (anhydrous)
cream-coloured solid (hexahydrate) |
| Density | 4.46 g/cm3 (anhydrous)
2.383 g/cm3 (hexahydrate) |
| Melting point | 682 °C (1,260 °F; 955 K) |
| Boiling point | decomposes |
| 92.4 g/100 mL (10 °C) | |
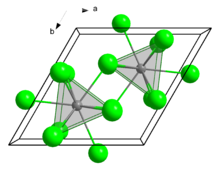
| Structure | |
| hexagonal, hP8 | |
| P63/m, No. 176 | |
| Tricapped trigonal prismatic (nine-coordinate) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319 | |
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |
| Related compounds | |
Other anions
|
Samarium(III) fluoride Samarium(III) bromide Samarium(III) oxide |
Other cations
|
Samarium(II) chloride Promethium(III) chloride Europium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O.[1] The compound has few practical applications but is used in laboratories for research on new compounds of samarium.
- ^ F. T. Edelmann, P. Poremba (1997). W. A. Herrmann (ed.). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. 6. Stuttgart: Georg Thieme Verlag.