
Back Selbstorganisierende Monoschicht German Αυτοσυναρμολογούμενες μονοστιβάδες Greek Monocapa autoensamblada Spanish تک لایههای خودآرا Persian Monostrato auto-assemblato Italian 自己組織化単分子膜 Japanese Самособирающиеся монослои Russian
| Part of a series of articles on |
| Molecular self-assembly |
|---|
 |
Self-assembled monolayers (SAM) are assemblies of organic molecules that form spontaneously on surfaces by adsorption and organize themselves into more or less distinct domains (head group, chain/backbone, and tail/end group).[1][2] In some cases, molecules that form the monolayer do not interact strongly with the substrate. This is the case for porphyrins on HOPG[3] and two-dimensional supramolecular networks[4] of PTCDA on gold[5]. In other cases, the head group has a strong affinity for the substrate and anchors the molecule.[6] Such an SAM consisting of a head group, chain (labeled "tail"), and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, and phosphonates.
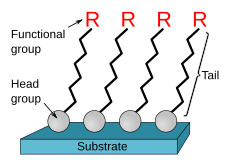
SAMs are created by the chemisorption of head groups onto a substrate from either the vapor or liquid phase[7][8] followed by a slower organization of "tail groups".[9] Initially, at small molecular density on the surface, adsorbate molecules form either a disordered mass of molecules or an ordered two-dimensional "lying down phase".[7] At higher molecular coverage, adsorbates can begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface over a period of minutes to hours.[10] The head groups assemble on the substrate, while the tail groups assemble far from the substrate. Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.
Adsorbate molecules adsorb readily because they lower the surface free-energy of the substrate[1] and are stable due to the strong chemisorption of the head groups. These bonds create monolayers that are more stable than the physisorbed bonds of Langmuir–Blodgett films.[11][12] For example, the trichlorosilane head group of an FDTS molecule reacts with a hydroxyl group on a substrate to form a very stable covalent bond [R-Si-O-substrate] with an energy of 452 kJ/mol.[citation needed] Thiol-metal bonds are on the order of 100 kJ/mol, making them fairly stable in a variety of temperatures, solvents, and potentials.[10] Monolayers pack tightly due to van der Waals interactions,[1][12] thereby reducing their own free energy.[1] The adsorption can be described by the Langmuir adsorption isotherm if lateral interactions are neglected. If they cannot be neglected, the adsorption is better described by the Frumkin isotherm.[10]
- ^ a b c d Love; et al. (2005). "Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology". Chem. Rev. 105 (4): 1103–1170. doi:10.1021/cr0300789. PMID 15826011.
- ^ Barlow, S.M.; Raval R.. (2003). "Complex organic molecules at metal surfaces: bonding, organisation and chirality". Surface Science Reports. 50 (6–8): 201–341. Bibcode:2003SurSR..50..201B. doi:10.1016/S0167-5729(03)00015-3.
- ^ De Feyter, S.; De Schreyer F.C. (2003). "Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy". Chemical Society Reviews. 32 (3): 139–150. CiteSeerX 10.1.1.467.5727. doi:10.1039/b206566p. PMID 12792937.
- ^ Elemans, J.A.A.W.; Lei S., De Feyter S. (2009). "Molecular and Supramolecular Networks on Surfaces: From Two-Dimensional Crystal Engineering to Reactivity". Angew. Chem. Int. Ed. 48 (40): 7298–7332. doi:10.1002/anie.200806339. hdl:2066/75325. PMID 19746490.
- ^ Witte, G.; Wöll Ch. (2004). "Growth of aromatic molecules on solid substrates for applications in organic electronics". Journal of Materials Research. 19 (7): 1889–1916. Bibcode:2004JMatR..19.1889W. doi:10.1557/JMR.2004.0251.
- ^ Carroll, Gregory T.; Pollard, Michael M.; van Delden, Richard A.; Feringa, Ben L. (2010). "Controlled rotary motion of light-driven molecular motors assembled on a gold film" (PDF). Chem. Sci. 1 (1): 97–101. doi:10.1039/C0SC00162G. S2CID 97346507.
- ^ a b Schwartz, D.K., Mechanisms and Kinetics of Self-Assembled Monolayer Formation (2001). "Mechanisms and kinetics of self-assembled monolayer formation". Annu. Rev. Phys. Chem. 52: 107–37. Bibcode:2001ARPC...52..107S. doi:10.1146/annurev.physchem.52.1.107. PMID 11326061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schreiber, F (30 November 2000). "Structure and growth of self-assembling monolayers". Progress in Surface Science. 65 (5–8): 151–257. Bibcode:2000PrSS...65..151S. doi:10.1016/S0079-6816(00)00024-1.
- ^ Wnek, Gary, Gary L. Bowlin (2004). Encyclopedia of Biomaterials and Biomedical Engineering. Informa Healthcare. pp. 1331–1333.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Vos, Johannes G., Robert J. Forster, Tia E. Keyes (2003). Interfacial Supramolecular Assemblies. Wiley. pp. 88–94.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Madou, Marc (2002). Fundamentals of Microfabrication: The Science of Miniaturization. CRC. pp. 62–63.
- ^ a b Kaifer, Angel (2001). Supramolecular Electrochemistry. Coral Gables. Wiley VCH. pp. 191–193.