Back TRPV1 BS TRPV1 Czech TRPV1 Welsh Transient Receptor Potential Vanilloid 1 German TRPV1 Spanish TRPV1 Persian TRPV1 French TRPV1 Japanese TRPV1 Korean TRPV1 Ukrainian
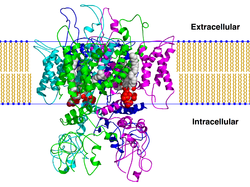
The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group.[5][6] This protein is a member of the TRPV group of transient receptor potential family of ion channels.[7] Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA).[8] The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1[9][10] (a chemical irritant receptor) to mediate the detection of noxious environmental stimuli.[11]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000196689 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000005952 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (October 1997). "The capsaicin receptor: a heat-activated ion channel in the pain pathway". Nature. 389 (6653): 816–824. Bibcode:1997Natur.389..816C. doi:10.1038/39807. PMID 9349813. S2CID 7970319.
- ^ Xue Q, Yu Y, Trilk SL, Jong BE, Schumacher MA (August 2001). "The genomic organization of the gene encoding the vanilloid receptor: evidence for multiple splice variants". Genomics. 76 (1–3): 14–20. doi:10.1006/geno.2001.6582. PMID 11549313.
- ^ Clapham DE, Julius D, Montell C, Schultz G (December 2005). "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels". Pharmacological Reviews. 57 (4): 427–450. doi:10.1124/pr.57.4.6. PMID 16382100. S2CID 17936350.
- ^ McPartland JM (2004-04-01). "Phylogenomic and chemotaxonomic analysis of the endocannabinoid system". Brain Research Reviews. 45 (1): 18–29. doi:10.1016/j.brainresrev.2003.11.005. ISSN 0165-0173. PMID 15063097. S2CID 25038370.
- ^ Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D (April 2015). "Structure of the TRPA1 ion channel suggests regulatory mechanisms". Nature. 520 (7548): 511–517. Bibcode:2015Natur.520..511P. doi:10.1038/nature14367. PMC 4409540. PMID 25855297.
- ^ Zhao J, Lin King JV, Paulsen CE, Cheng Y, Julius D (September 2020). "Irritant-evoked activation and calcium modulation of the TRPA1 receptor". Nature. 585 (7823): 141–145. Bibcode:2020Natur.585..141Z. doi:10.1038/s41586-020-2480-9. PMC 7483980. PMID 32641835. S2CID 220407248.
- ^ Basbaum AI, Bautista DM, Scherrer G, Julius D (October 2009). "Cellular and molecular mechanisms of pain". Cell. 139 (2): 267–284. doi:10.1016/j.cell.2009.09.028. PMC 2852643. PMID 19837031.