
Back فلوريد القصدير الرباعي Arabic تترافلوئورید تنهکه AZB Fluorid cíničitý Czech Zinn(IV)-fluorid German Fluoruro de estaño(IV) Spanish تترافلوئورید قلع Persian Tina(IV)fluoridi Finnish Tetrafluoruro di stagno Italian フッ化スズ(IV) Japanese Фторид олова(IV) Russian
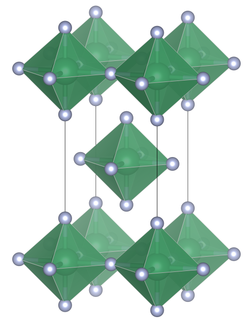 Unit cell of tin(IV) fluoride
| |
| Names | |
|---|---|
| IUPAC name
tin(IV) fluoride
| |
| Other names
stannic fluoride, tin tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.105 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| SnF4 | |
| Molar mass | 194.704 g/mol |
| Appearance | white solid |
| Density | 4.78 g / cm3 |
| Melting point | above 700 °C (sublimes) |
| Structure | |
| Tetragonal, tI10 | |
| I4/mmm, No. 139 | |
| Related compounds | |
Other anions
|
Tin(IV) chloride Tin(IV) bromide Tin(IV) iodide |
Other cations
|
Carbon tetrafluoride Silicon tetrafluoride Germanium tetrafluoride Tin tetrafluoride Lead tetrafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4. It is a white solid. As reflected by its melting point above 700 °C, the tetrafluoride differs significantly from the other tetrahalides of tin.[1]
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford:Butterworth-Heinemann. pp. 381. ISBN 0-7506-3365-4.