
Back تريميبرامين Arabic تریمیپرامین AZB Trimipramin Welsh Trimipramin German Trimipramina Spanish تریمیپرامین Persian Trimipramiini Finnish Trimipramine French Trimipramina Italian Trimipraminum Latin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Surmontil, others |
| Other names | Trimeproprimine; IF-6120; IL-6001; RP-7162; 2'-Methylimipramine; β-Methylimipramine |
| AHFS/Drugs.com | |
| MedlinePlus | a602010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral, intramuscular injection, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 41%[2][3][4][5] |
| Protein binding | 94.9%[2][3][4][5] |
| Metabolism | Hepatic[2][3][4][5] |
| Elimination half-life | 23–24 hours[2][3][4][5] |
| Excretion | Renal[2][3][4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.917 |
| Chemical and physical data | |
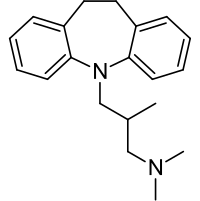
| Formula | C20H26N2 |
| Molar mass | 294.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression.[6][7][8][9] It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively.[6][7][8][9] The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor.[10] Similarly to other TCAs, however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.[6][7][8][9]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c d e "PRODUCT INFORMATION SURMONTIL Tablets and Capsules" (PDF). TGA eBusiness Services. Aspen Pharmacare Australia Pty Ltd. 28 November 2012. Retrieved 30 November 2013.
- ^ a b c d e "SURMONTIL (trimipramine maleate) capsule [Duramed Pharmaceuticals Inc]". DailyMed. Duramed Pharmaceuticals Inc. December 2012. Retrieved 30 November 2013.
- ^ a b c d e "Surmontil, Trimip (trimipramine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 30 November 2013.
- ^ a b c d e "Trimipramine 50mg Capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Zentiva. 19 November 2012. Retrieved 30 November 2013.
- ^ a b c Berger M, Gastpar M (1996). "Trimipramine: a challenge to current concepts on antidepressives". Eur Arch Psychiatry Clin Neurosci. 246 (5): 235–9. doi:10.1007/bf02190274. PMID 8863001. S2CID 29596291.
- ^ a b c Gastpar M (1989). "Clinical originality and new biology of trimipramine". Drugs. 38 (Suppl 1): 43–8, discussion 49–50. doi:10.2165/00003495-198900381-00010. PMID 2693055. S2CID 23302529.
- ^ a b c Pecknold JC, Luthe L (1989). "Trimipramine, anxiety, depression and sleep". Drugs. 38 (Suppl 1): 25–31, discussion 49–50. doi:10.2165/00003495-198900381-00007. PMID 2693052. S2CID 20347877.
- ^ a b c Lapierre YD (1989). "A review of trimipramine. 30 years of clinical use". Drugs. 38 (Suppl 1): 17–24, discussion 49–50. doi:10.2165/00003495-198900381-00006. PMID 2693051. S2CID 22227558.
- ^ Cite error: The named reference
pmid9090573was invoked but never defined (see the help page).