
Back Uraan(VI)fluoried Afrikaans سداسي فلوريد اليورانيوم Arabic هقزافلوراید اورانیوم AZB Hexafluorur d'urani Catalan Fluorid uranový Czech Uran(VI)-fluorid German Hexafluoruro de uranio Spanish Uranio hexafluoruro Basque هگزافلوراید اورانیوم Persian Uraaniheksafluoridi Finnish
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Uranium hexafluoride
Uranium(VI) fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | hex |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.116 |
| EC Number |
|
| 2923 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2978 (<1% 235U) 2977 (>1% 235U) |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| UF6 | |
| Molar mass | 352.02 g/mol |
| Appearance | Colorless solid |
| Density | 5.09 g/cm3, solid |
| Boiling point | 56.5 °C (133.7 °F; 329.6 K) (sublimes, at atmospheric pressure) |
| Hydrolyzes | |
| Solubility |
|
| Structure | |
| Orthorhombic, oP28 | |
| Pnma, No. 62 | |
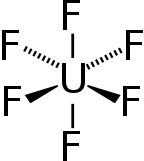
| Octahedral (Oh) | |
| 0 | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
|
Std enthalpy of
formation (ΔfH⦵298) |
|
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, corrosive, radioactive[3] |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H330, H373, H411 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1250 |
| Related compounds | |
Other anions
|
Uranium hexachloride |
Other cations
|
|
Related uranium fluorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile, toxic white solid that is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.[4]
- ^ "Uranium Hexafluoride". Archived from the original on 2013-09-16. Retrieved 2013-08-08.
- ^ a b c d Johnson, Gerald K. (1979). "The Enthalpy of Formation of Uranium Hexafluoride". The Journal of Chemical Thermodynamics. 11 (5): 483–490. doi:10.1016/0021-9614(79)90126-5.
- ^ Uranium(VI) fluoride
- ^ Cite error: The named reference
Ullwas invoked but never defined (see the help page).
