
Back سلفاديميدين Arabic Sulfadimidin German Sulfadimidine English سولفادیمیدین Persian Sulfadimidiini Finnish Sulfadimidina Italian スルファジミジン Japanese Sulfadimidină Romanian Sulfadimidin Serbo-Croatian Sulfadimidin Serbian
| |
|---|---|
| Nama sistematis (IUPAC) | |
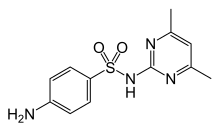
| 4-amino-N-(4,6-dimetilpirimidin-2-il) benzena-1-sulfonamida | |
| Data klinis | |
| AHFS/Drugs.com | International Drug Names |
| Kat. kehamilan | ? |
| Status hukum | ? |
| Pengenal | |
| Nomor CAS | 57-68-1 |
| Kode ATC | J01EB03 QJ01EQ03 QP51AG01 QP51AG51 |
| PubChem | CID 5327 |
| DrugBank | DB01582 |
| ChemSpider | 5136 |
| UNII | 48U51W007F |
| KEGG | D02436 |
| ChEBI | CHEBI:102265 |
| ChEMBL | CHEMBL446 |
| NIAID ChemDB | AIDSNO:027749 |
| Data kimia | |
| Rumus | C12H14N4O2S |
| SMILES | eMolecules & PubChem |
| |
| Data fisik | |
| Titik lebur | 176 °C (349 °F) |
Sulfadimidin atau sulfametazin adalah salah satu antibiotik sulfonamida.
Ada singkatan yang tidak terstandarisasia untuknya seperti "sulfadimidin" (disingkat SDI[1][2] dan lebih umum tetapi kurang dapat diandalkanb SDD[3][4]) dan sebagai "sulfametazin" (disingkat SMT[5][6] dan lebih umum tetapi kurang dapat diandalkanc SMZ[7][8]).
- ^ Romváry A, Simon F (1992). "Sulfonamide residues in eggs". Acta Veterinaria Hungarica. 40 (1–2): 99–106. PMID 1476095.
- ^ Reddy KS, Jain SK, Uppal RP (1988). "Pharmacokinetic studies of sulphonamides in poultry". Indian Journal of Animal Sciences.
- ^ Kamakura K, Hasegawa M, Koiguchi S, Miyata M, Okamoto K, Narita M, et al. (1993). "[Studies on the identification of sulfadimidine in pork by high performance liquid chromatography with photodiode array detector and gas chromatograph-mass spectrometry]". Eisei Shikenjo Hokoku. Bulletin of National Institute of Hygienic Sciences (111): 61–5. PMID 7920569.
- ^ Garg SK, Ghosh SS, Mathur VS (January 1986). "Comparative pharmacokinetic study of four different sulfonamides in combination with trimethoprim in human volunteers". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 24 (1): 23–5. PMID 3485584.
- ^ Peña MS, Salinas F, Mahedero MC, Aaron JJ (February 1994). "Solvent effect on the determination of sulfamethazine by room-temperature photochemically induced fluorescence". Talanta. 41 (2): 233–6. doi:10.1016/0039-9140(94)80113-4. PMID 18965913.
- ^ Kaniou S, Pitarakis K, Barlagianni I, Poulios I (July 2005). "Photocatalytic oxidation of sulfamethazine". Chemosphere. 60 (3): 372–80. Bibcode:2005Chmsp..60..372K. doi:10.1016/j.chemosphere.2004.11.069. PMID 15924956.
- ^ Calvo R, Sarabia S, Carlos R, Du Souich P (Mar 1987). "Sulfamethazine absorption and disposition: effect of surgical procedures for gastroduodenal ulcers". Biopharmaceutics & Drug Disposition. 8 (2): 115–24. doi:10.1002/bdd.2510080203. PMID 3593892.
- ^ De Liguoro M, Fioretto B, Poltronieri C, Gallina G (June 2009). "The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim". Chemosphere. 75 (11): 1519–24. Bibcode:2009Chmsp..75.1519D. doi:10.1016/j.chemosphere.2009.02.002. PMID 19269673.