| Epanolol
|
|
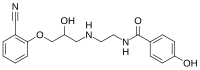
| IUPAC ime
|
| |
|---|
| (RS)-N-[2-([3-(2-cijanofenoksi)-2-hidroksipropil]amino)etil]-2-(4-hidroksifenil)acetamid |
|
| Identifikacija
|
| CAS registarski broj
|
86880-51-5  Y Y
|
| PubChem[1][2]
|
72014
|
| KEGG[3]
|
D06646
|
| ATC code
|
C07AB10
|
| Jmol-3D slike
|
Slika 1
|
|
|---|
C1=CC=C(C(=C1)C#N)OCC(CNCCNC(=O)CC2=CC=C(C=C2)O)O |
|
|
|---|
InChI=1S/C20H23N3O4/c21-12-16-3-1-2-4-19(16)27-14-18(25)13-22-9-10-23-20(26)11-15-5-7-17(24)8-6-15/h1-8,18,22,24-25H,9-11,13-14H2,(H,23,26)  Y Y
Kod: YARKMNAWFIMDKV-UHFFFAOYSA-N  Y Y |
|
| Svojstva
|
| Molekulska formula
|
C20H23N3O4
|
| Molarna masa
|
369,41432
|
|
 Y (šta je ovo?)
(verifikuj) Y (šta je ovo?)
(verifikuj)
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala
|
| Infobox references
|
Epanolol je beta blokator.[4]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Hosie, J.; Scott, A. K.; Petrie, J. C.; Cockshott, I. D. (1990). „Pharmacokinetics of epanolol after acute and chronic oral dosing in elderly patients with stable angina pectoris”. British Journal of Clinical Pharmacology 29 (3): 333–337. PMC 1380134. PMID 1968755.

