
Back Doppelschichtkapazität German Double-layer capacitance English Ємність подвійного шару Ukrainian
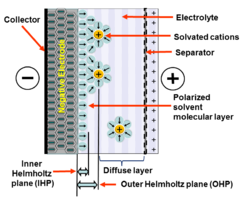
Double-layer capacitance is the storing of electrical energy by means of the electrical double layer effect.[1][2]
Double layer capacitance is when an electrode and a liquid solution are touching each other, causing the charges to line up and allowing electricity to be stored there. The double layer is created when the electrode’s surface is charged through the application of electricity. This causes oppositely charged molecules to be attracted to the surface (since opposites attract). This first layer is well attached to the electrode surface, causing it to be semi-permanent. The second layer (with opposite charge) is held by this first layer, making it less attached to the electrode than the first layer. These two layers are separated by a single atomic layer of uncharged molecules in the solution. These alternating layers of charges have the ability to store electrical energy, in a way that depends on the amount of electricity initially applied to the electrode. Once this charge is stored in the device, the source of electricity can be removed and the circuit can still be powered from this stored electricity contained in this device, until it is used up. [3]

The amount of electric charge stored in double-layer capacitance is linearly proportional to the applied voltage. It depends mainly on the electrode surface. The unit of capacitance is the farad.
- ↑ Z. Stojek, The electrical double layer and its structure
- ↑ "The electrical double layer". 2011. Archived from the original on 2011-05-31. Retrieved 2014-01-20.
- ↑ Z. Stojek, The Electrical Double Layer and Its Structure