
Back راملتيون Arabic Ramelteon German Ramelteon English راملتئون Persian Ramelteon French Ramelteon Italian ラメルテオン Japanese Ramelteon Dutch ରାମେଲଟେଅନ OR Ramelteon Polish
Ramelteon is a central nervous system (CNS) depressant medication that can help people sleep. It is used for patients that have trouble falling asleep.
Ramelteon has significant drug-drug interaction with the following drugs: amiodarone[*], ciprofloxacin[*], fluvoxamine[*], ticlopidine[*].
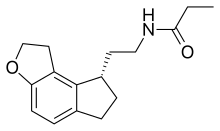 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rozerem, others |
| Synonyms | TAK-375 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605038 |
| License data | |
| Dependence liability | Low[1] |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 1.8%[3] |
| Protein binding | 82% (mainly albumin)[3] |
| Metabolism | Liver (CYP1A2 major, CYP2C and CYP3A4 minor)[3] |
| Metabolites | M-II (active metabolite)[3] |
| Elimination half-life | Ramelteon: 1–2.6 hours[3] M-II: 2–5 hours[3][4] |
| Excretion | Kidney: 84%[3] Feces: 4%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.215.666 |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
- ↑ Kim, HK; Yang, KI (December 2022). "Melatonin and melatonergic drugs in sleep disorders". Translational and Clinical Pharmacology. 30 (4): 163–171. doi:10.12793/tcp.2022.30.e21. PMC 9810491. PMID 36632077.
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
{{cite web}}: line feed character in|title=at position 4 (help) - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Cite error: The named reference
Rozerem FDA labelwas used but no text was provided for refs named (see the help page). - ↑ Karim A, Tolbert D, Cao C (February 2006). "Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia". Journal of Clinical Pharmacology. 46 (2): 140–148. doi:10.1177/0091270005283461. PMID 16432265. S2CID 38171735.