Back 1,2-Dioxin German 1,2-Dioxin English 1,2-Dioxin Hungarian 1,2-Dioksin ID 1,2-diossina Italian 1,2-ジオキシン Japanese 1,2-Dioksin Serbo-Croatian 1,2-டையாக்சின் Tamil 1,2-二噁英 Chinese
| |||
| Nazivi | |||
|---|---|---|---|
| Sistemski IUPAC naziv
1,2-Dioksin[1] | |||
| Identifikacija Error in template * unknown parameter name (Template:Chembox Identifiers-lat): "PubChem_Ref" (В. списак параметара). Ова грешка је безопасна. Порука се приказује само кад се користи дугме Прикажи претпреглед; неће бити приказана након што се притисне Објави измену.
| |||
3D model (Jmol)
|
|||
| ChemSpider | |||
| |||
| Svojstva Error in template * unknown parameter name (Template:Chembox Properties-lat): "ExactMass" (В. списак параметара). Ова грешка је безопасна. Порука се приказује само кад се користи дугме Прикажи претпреглед; неће бити приказана након што се притисне Објави измену.
| |||
| C4H4O2 | |||
| Molarna masa | 84,07 g·mol−1 | ||
| Srodna jedinjenja | |||
Srodna jedinjenja
|
Dibenzodioksin | ||
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |||
| Reference infokutije | |||
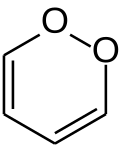
1,2-Dioksin je heterociklično, organsko, antiaromatično jedinjenje sa hemijskom formulom C4H4O2. On je izomerna forma 1,4-dioksina (ili p-dioksina).
Usled njegovih peroksidu sličnih karakteristika, 1,2-dioksin je veoma nestabilan i ne može se izolovati. Čak su i supstituisani derivati su veoma nestabilni, e.g. 1,4-difenil-2,3-benzodioksin.[4] 3,6-bis(p-tolil)-1,2-dioksin je bio pogrešno proglašen prvim stabilnim derivatom 1990. godine.[5][6]
-
Izomeri 1,2-dioksina (levo) i 1,4-dioksina (desno)
-
Struktura prelaznog 1,4-difenil-2,3-benzodioksina
-
Dioksinska (1) i dionska forma (2)
- ^ „CID 15559065 - PubChem Public Chemical Database”. The PubChem Project. USA: National Center for Biotechnology Information. Descriptors Computed from Structure.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Smith, Jimmie P.; Schrock, Alan K.; Schuster, Gary B. (1982). „Chemiluminescence of organic peroxides. Thermal generation of an o-xylylene peroxide”. Journal of the American Chemical Society. 104 (4): 1041—1047. doi:10.1021/ja00368a021.
- ^ Shine, Henry J.; Zhao, Da Chuan (1990). „Electron transfer to excited doublet states. Photoirradiation of 10-methylphenothiazine cation radical perchlorate in solutions of phenylacetylene and p-tolylacetylene in acetonitrile”. The Journal of Organic Chemistry. 55 (13): 4086—4089. doi:10.1021/jo00300a026.
- ^ Block, Eric; Shan, Zhixing; Glass, Richard S.; Fabian, Jürgen (2003). „Revised Structure of a Purported 1,2-Dioxin: A Combined Experimental and Theoretical Study”. The Journal of Organic Chemistry. 68 (10): 4108—4111. PMID 12737603. doi:10.1021/jo034305i.